- Home - News
- TWI News | TV
- Polls
- Year In Review
- News Archive
- Crime & Punishment
- Politics
- Regional
- Editorial
- Health
- Ghanaians Abroad
- Tabloid
- Africa
- Religion
- Election 2020
- Coronavirus
- News Videos | TV
- Photo Archives
- News Headlines
- Press Release
General News of Monday, 31 January 2011
Source: The Herald
Herbal Drugs Causing Heart & Kidney Failures
Reports from some of the nation’s leading hospitals indicate that herbal medicines are causing life- threatening medical complications, including kidney, heart failures, strokes, various cancers and, sometimes, even death.
The Komfo Anokye Kidney Centre in Kumasi and the Saint Joseph’s Hospital, Koforidua, are reported to have witnessed increasing cases of health complications resulting from continuous use of herbal medicines whose efficacy and toxicity have not been confirmed, yet are in the market for human consumption.
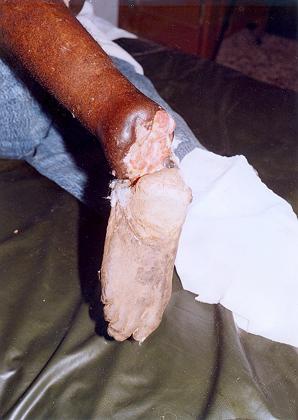
There have been instances in Komfo Anokye Hospital and the Saint Joseph’s Hospital where two persons have had to be amputated, one in the leg and the other in the arm, after continuous use of unapproved herbal concoctions, leading to their arm and leg respectively rotten.
Diabetes patients have had to suffer rotten legs, after they were convinced by herbal medicine producers to turn to the use of their drugs and stop taking recommended medicine from the hospital. When they complied, they fell into diabetes- induced coma, and their legs had to be cut off in the hospitals to save their lives. Others have had to die on the operation table.
This, according to health experts, is because there have not been any serious studies to find out whether the herbal concoctions become poisonous (toxic) or have side effects in the long-term. The Mampong Center for Scientific Research into Plant Medicine which has its seal on lots of the herbal medicines only conducts tests on toxicity of herbal medicines in the short-term.
It is in the light of this that the President of the Pharmaceutical Manufacturers Association of Ghana (PMAG), Dr. Michael Agyekum Addo (KAMA), has commended the Food and Drugs Board and its chief executive on their attempts at ensuring sanity in the herbal drug production.
He has noted that herbal medicines need to be regulated as people react to even natural produce such as vegetables.
He is also of the view that once herbalists go into commercial production, they are into pharmaceuticals, and therefore, would require training on formulation.
They also need training on how to package the products since “not all medicines could stand plastics or glass bottles, especially in different colours, since some products are sensitive to light”, The Daily Guide last week reported him as saying.
He, therefore, sees what FDB is seeking to do as reforming and bringing some kind of standardization into the system where persons who produce medicine, be it orthodox or herbal, would have to conform.
He said that this would ensure that products which get to consumers are of high quality and not inferior or expired. Whilst advising herbalists not to see the FDB as a monstrous organization with the aim of running their businesses down, he pointed out that it was doing its mandatory work.
The FDB, he added, has the right to regulate food and drugs in the country to ensure that, only the best of products get onto the Ghanaian market.
Dr. Agyekum Addo is also the Chief Executive Officer of KAMA Industries, a pharmaceutical company, which also produces drugs for local consumption.
It is learned that the Ghana Medical Association also plans to issue a statement in support of Dr. Opuni’s efforts at ensuring that whatever drugs come out of the herbal manufacturing companies are safe for consumption.
Some herbal medicines are formulated on large-scale in unapproved premises and under very unhygienic conditions, by unqualified people. Sometimes, one herbal product has multiplicity of claims.
The Faculty of Pharmacy of KNUST, Department of Pharmacology of University of Ghana Medical School and the Nouguchi Memorial Medical Centre for Research do toxicological studies of herbal medicine to prove their long-term toxicity and efficacy. But investigations have revealed that, the herbalists don’t go to them for their services; they claim that the fees charged by these institutions are unbearable.
Officials at the Food and Drugs Board argue that if the efficacy studies and chronic (long-term) toxicological studies are done, the genuine herbalists could have their herbal products included on the essential medicines list of the Ministry of Health; they could also export their products to other countries for foreign exchange.
To ensure that herbal medicines are safe and efficacious, the Food and Drugs Board (FDB) is only asking for chronic (long-term) toxicological studies and efficacy studies reports on herbal medicines.
Although the herbalists have claimed that their medicines can treat all types of diseases including headache, hypertension, diabetes, Asthma, piles (Kooko), hernia, and infertility, these claims can’t be true. The claims are ridiculous as conditions like hernia and fibroid can only be treated by surgery.
There is no doubt about the medicinal values of some plants because, according to some medical doctors and pharmacists, the active pharmaceutical ingredients of some orthodox medicines are extracted from plants.
However, over the past ten years herbal centres and herbal clinics have proliferated like mushrooms in the country and are producing questionable products.
Most of the herbal centres, according to investigations, are managed by unqualified practitioners who dispense to patients unregistered and unwholesome herbal medicines.
Meanwhile, the European Union is set to place a ban on all herbal remedies and drugs in the United Kingdom.
The ban is expected to begin in May this year, and traditional herbal medicinal products would be expected to be licensed or prescribed by a registered herbal practitioner in conformity with an EU directive passed in 2004.
The recent action was triggered by the fact that there had been an ever rising concern over adverse effects caused by herbal medicines. The UK Medicines and Healthcare Products Regulatory Agency (MHRA) has issued more than a dozen safety alerts in the past two years owing to various complaints by people who consumed such herbal drugs.
According to the Alliance for Natural Health (ANH), the representative body for herbal practitioners, not a single product used in traditional Chinese medicine or ayurvedic medicine has been licensed, the UK Independent Newspaper cited.
This could mean close to 2500 UK qualified herbalists and Chinese medicine practitioners will lose the right to supply a wide-range of herbal medicines, because they are not signed up to the statutory regulation scheme.
However, natural practitioners also add in chorus that the costs of obtaining licenses are “way beyond their means.”
But the ANH is quick to counter that argument, saying that the cost of obtaining a license ranges between GBP 80,000 to GBP 120,000 per herb, which it says is “very much affordable”.
Michael McIntyre, the chairman of the European Herbal and Traditional Medicine Practitioners Association, said: “The problem is you can’t get a license for many herbal medicines because they are grown in people’s back gardens and you can’t patent them.
“The implications are very serious. Patients want to receive treatment from trained and qualified practitioners but unless we have regulation they can’t have confidence in who is treating them.
“The worst outcome is that patients will end up going to the internet for their herbal medicines where there are no controls,” he said.