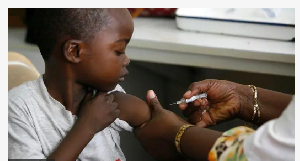
Di first malaria treatment wey dey suitable for babies and very young children don dey approved for use.
Di drug, wey dey known as Coartem Baby or Riamet Baby for some countries, bin dey developed by Novartis in collaboration with di Medicines for Malaria Venture (MMV), wey be a Swiss-based not-for-profit organisation wey bin first get backing from di British, Swiss and Dutch Governments, as well as di World Bank and di Rockefeller Foundation.
E dey expected for roll out for African countries in weeks.
Until now, no approved malaria drugs dey specifically for babies.
Instead, dem dey treat dem with di ones wey dey formulated for older children wey involve a risk of overdose.
Half a million deaths for 2023
For 2023 - di year wey di most recent figures dey available - malaria bin dey linked to around 597,000 deaths.
Almost all of di deaths bin dey for Africa, and around three quarters of dem na children under five years old.
Malaria treatments for children dey, but until now, no one dey specifically for di very youngest babies and small children, wey weigh less dan 4.5kg or around 10lb.
Instead, dem dey treat dem with drugs wey dey designed for older children.
But e involve risks, as doses for dis older children fit no dey safe for babies, wey dia liver functions still dey develop and dia body dey process medicine differently.
Experts say dis don lead to wetin dey described as a "treatment gap".
Now, dis new medicine, developed by di drug company Novartis, don dey approved by di Swiss authorities and e dey likely to dey rolled out in regions and countries with di highest rates of malaria within weeks.
Novartis dey plan to introduce am on a largely not-for-profit basis.
Di smallest and most vulnerable
Di company chief executive, Vas Narasimhan, say dis na important moment.
"For more dan three decades, we don dey chook eye in di fight against malaria, working relentlessly to deliver scientific breakthroughs wia dem dey needed most.
"Togeda with our partners, we dey proud to done go further to develop di first clinically proven malaria treatment for newborns and young babies, ensuring say even di smallest and most vulnerable fit finally receive di care dem deserve."
Eight African nations also take part in di assessment and trials of di drug and dem dey expected to be among di first to access am.
Martin Fitchet, CEO of MMV, say, dis na important step on di road towards ending di ogbonge palava of malaria.
"Malaria na one of di world deadliest diseases, particularly among children. But wit di right resources and focus, E fit dey eliminated.
"Di approval of Coartem Baby provide a necessary medicine wit optimised dose to treat a neglected group of patients and offer a valuable addition to di antimalarial toolbox."
Dr Marvelle Brown, associate professor for di University of Hertfordshire School of Health, Medicine and Life Sciences, say dis suppose dey seen as a major breakthrough in saving di lives of babies and young children.
"Di death rate for malarial infections, particularly for sub-Saharan Africa dey very high - over 76% of deaths dey hapun in children under five years old.
"Increase in death from malaria dey further increased in babies born with sickle cell disease, primarily due to a weak immune system.
"From a public health perspective, Novartis making dis not-for-profit fit help wit reducing inequality in access to healthcare."
BBC Pidgin of Tuesday, 8 July 2025
Source: BBC